100 gm bar:
৳ 690.00
Indications
Shampoo: Ketozole shampoo is indicated for the treatment and prophylaxis of infections in which the yeast Malassezia (previously called Pityrosporum) is involved, such as pityriasis versicolor (localized), seborrhoeic dermatitis and pityriasis capitis (dandruff).
Cream: Cream is used for topical application in the treatment of dermatophyte infections of the skin such as tinea corporis, tinea cruris (dhobie itch), tinea manus and tinea pedis (athlete’s foot) infections due to Trichophyton spp, Microsporon spp and Epidermophyton spp. Ketozole 2% cream is also indicated for the treatment of cutaneous candidosis (including vulvitis), candidal intertrigo (sweat rash), tinea (pityriasis) versicolor and seborrhoeic dermatitis caused by Malassezia (previously called Pityrosporum) spp.
Tablet: Treatment of superficial and deep mycoses:
Prophylactic treatment to prevent mycotic infection in patients with reduced host defenses, e.g., patients with cancer, organ transplant and burns.
Medicated bar: Medicated bar is indicated for All Kinds of Body Fungus.
Cream: Cream is used for topical application in the treatment of dermatophyte infections of the skin such as tinea corporis, tinea cruris (dhobie itch), tinea manus and tinea pedis (athlete’s foot) infections due to Trichophyton spp, Microsporon spp and Epidermophyton spp. Ketozole 2% cream is also indicated for the treatment of cutaneous candidosis (including vulvitis), candidal intertrigo (sweat rash), tinea (pityriasis) versicolor and seborrhoeic dermatitis caused by Malassezia (previously called Pityrosporum) spp.
Tablet: Treatment of superficial and deep mycoses:
- Infections of the skin, hair and nails by dermatophytes and/or yeasts (dermatomycosis, onychomycosis, perionyxis, pityriasis versicolor, chronic mucocutaneous candidiasis etc.) especially when topical treatment is difficult or not very effective, owing to involvement of large skin surfaces or to lesions affecting deeper dermal layers, nails and hairs
- Yeast infection of the mouth (oral thrush, perleche) and the gastrointestinal tract
- Vaginal candidiasis, especially chronic recurrent cases or cases responding poorly to topcial treatment
- Systemic mycotic infections such as systemic candidiasis, paracoccidioidomycosis, histoplasmosis, coccidioidomycosis etc.
Prophylactic treatment to prevent mycotic infection in patients with reduced host defenses, e.g., patients with cancer, organ transplant and burns.
Medicated bar: Medicated bar is indicated for All Kinds of Body Fungus.
Pharmacology
Ketoconazole interacts with 14-α-sterol demethylase, a cytochrome P-450 enzyme necessary for the conversion of lanosterol to ergosterol. This results in inhibition of ergosterol synthesis and increased fungal cellular permeability due to reduced amounts of ergosterol present in the fungal cell membrane. This metabolic inhibition also results in accumulation of 14α-methyl-3,6-diol, a toxic metabolite. The increase in membrane fluidity is also thought to produce impairment of membrane-bound enzyme systems as components become less closely packed.
Dosage & Administration
Shampoo: The affected areas of the skin or the scalp should be washed with ketoconazole 2% shampoo, which should be left on the skin/scalp for 3 to 5 minutes before rinsing.
Treatment:
Treatment:
- Pityriasis versicolor: Once daily for 5 days.
- Seborrhoeic dermatitis and pityriasis capitis: Twice weekly for 2 to 4 weeks.
- Pityriasis versicolor: Once daily for 3 days during a single treatment course before the summer.
- Seborrhoeic dermatitis and pityriasis capitis: Once every 1 or 2 weeks.
- Tinea pedis: Ketoconazole cream should be applied to the affected areas twice daily. The usual duration of treatment for mild infections is 1 week. For more severe or extensive infections (e.g. involving the sole or sides of the feet) treatment should be continued until a few days after all signs and symptoms have disappeared in order to prevent relapse.
- For other infections: Ketoconazole cream should be applied to the affected areas once or twice daily, depending on the severity of the infection. The treatment should be continued until a few days after the disappearance of all signs and symptoms. The usual duration of treatment is: tinea versicolor 2-3 weeks, tinea corporis 3-4 weeks. The diagnosis should be reconsidered if no clinical improvement is noted after 4 weeks. General measures in regard to hygiene should be observed to control sources of infection or reinfection. Seborrhoeic dermatitis is a chronic condition and relapse is highly likely.
- Vaginal candidiasis: 1 tablet (200 mg) tablet twice daily for 5 days.
- All other indications: 1 tablet (200 mg) once daily until at least one week after the symptoms have disappeared and the cultures have become negative.
- Pityriasis versicolor: 1 to 6 weeks
- Dermatomycoses: 2 to 8 weeks
- Onychomycoses: 1 to 12 months
- Mycoses of hair and scalp: 1 to 2 months
- Chronic mucocutaneous candidiasis : 1 to 12 months
- Oral mycoses: 5 to 10 days
- Systemic candidiasis: 1 to 2 months
- Paracoccidioidomycosis,histoplasmosis
- and other systemic mycosis: 1 month to 2 years
Interaction
Shampoo and Cream: No information was found.
Tablet: Reduced absorption with antimuscarinics, antacids, H2-blockers, PPIs and sucralfate. Reduced plasma concentrations with rifampicin, isoniazid, efavirenz, nevirapine, phenytoin. May also reduce concentrations of isoniazid and rifampicin. May reduce efficacy of oral contraceptives. May increase serum levels of CYP3A4 substrates e.g. digoxin, oral anticoagulants, sildenafil, tacrolimus.
Tablet: Reduced absorption with antimuscarinics, antacids, H2-blockers, PPIs and sucralfate. Reduced plasma concentrations with rifampicin, isoniazid, efavirenz, nevirapine, phenytoin. May also reduce concentrations of isoniazid and rifampicin. May reduce efficacy of oral contraceptives. May increase serum levels of CYP3A4 substrates e.g. digoxin, oral anticoagulants, sildenafil, tacrolimus.
Contraindications
Contraindicated in patients with known hypersensitivity to ketoconazole.
Side Effects
Shampoo: Topical treatment with Ketozole shampoo 2% is generally well tolerated. As with other shampoos, a local burning sensation, itching or contact dermatitis (due to irritation or allergy) may occur on exposed areas. Oily and dry hair have been reported rarely with the use of Ketozole shampoo 2%.
Cream: Commonly observed adverse reactions to Ketozole cream in clinical trials were skin application site burning sensation, erythema and pruritus. Uncommon adverse reactions are application site bleeding, discomfort, dryness, inflammation, irritation, paraesthesia and reaction; bullous eruption, dermatitis contact, rash, skin exfoliation and sticky skin.
Tablet: Ketozole is very well tolerated. Nausea and itching may occasionally occur. In some patients, an idosyncratic liver reaction may occur (incidence 1:10000).
Cream: Commonly observed adverse reactions to Ketozole cream in clinical trials were skin application site burning sensation, erythema and pruritus. Uncommon adverse reactions are application site bleeding, discomfort, dryness, inflammation, irritation, paraesthesia and reaction; bullous eruption, dermatitis contact, rash, skin exfoliation and sticky skin.
Tablet: Ketozole is very well tolerated. Nausea and itching may occasionally occur. In some patients, an idosyncratic liver reaction may occur (incidence 1:10000).
Pregnancy & Lactation
Shampoo: Since ketoconazole is not absorbed through the skin after topical application, pregnancy and lactation are not a contraindication for the use of ketoconazole shampoo 2%.
Cream: There are no adequate and well-controlled studies in pregnant or lactating women. To date, no other relevant epidemiological data are available. Data on a limited number of exposed pregnancies indicate no adverse effects of topical Ketoconazole on pregnancy or on the health of the foetus/newborn child. Animal studies have shown reproductive toxicity following oral administration of Ketoconazole. No effects on the breastfed newborn/infant are anticipated.
Tablet: Pregnancy category C. There is no adequate and well controlled studies in pregnant women. Ketoconazole Tablets should not be used during pregnancy and lactation.
Cream: There are no adequate and well-controlled studies in pregnant or lactating women. To date, no other relevant epidemiological data are available. Data on a limited number of exposed pregnancies indicate no adverse effects of topical Ketoconazole on pregnancy or on the health of the foetus/newborn child. Animal studies have shown reproductive toxicity following oral administration of Ketoconazole. No effects on the breastfed newborn/infant are anticipated.
Tablet: Pregnancy category C. There is no adequate and well controlled studies in pregnant women. Ketoconazole Tablets should not be used during pregnancy and lactation.
Precautions & Warnings
Shampoo: In patients who have been on prolonged treatment with topical corticosteroids, it is recommended that the steroid therapy be gradually withdrawn over a period of 2 to 3 weeks, while using Ketozole shampoo 2%, to prevent any potential rebound effect. Increased hair shedding is often associated with seborrhoeic dermatitis and dandruff, and has been rarely reported with the use of Ketozole shampoo 2%. Avoid contact with the eyes. If the shampoo should get into the eyes, they should be bathed with water.
Cream: Not for ophthalmic use. If a potent topical corticosteroid has been used previously in the treatment of seborrhoeic dermatitis, a recovery period of 2 weeks should be allowed before using Ketozole 2% w/w cream, as an increased incidence of steroid induced skin sensitisation has been reported when no recovery period is allowed.
Tablet: In patients with a previous history of liver disease, liver enzyme levels should be monitored during treatment. When patients develop symptoms indicative of liver reaction, such as nausea or fatigue, accompanied with pale faeces, dark urine or jaundice, Ketozole therapy should be stopped immediately
Cream: Not for ophthalmic use. If a potent topical corticosteroid has been used previously in the treatment of seborrhoeic dermatitis, a recovery period of 2 weeks should be allowed before using Ketozole 2% w/w cream, as an increased incidence of steroid induced skin sensitisation has been reported when no recovery period is allowed.
Tablet: In patients with a previous history of liver disease, liver enzyme levels should be monitored during treatment. When patients develop symptoms indicative of liver reaction, such as nausea or fatigue, accompanied with pale faeces, dark urine or jaundice, Ketozole therapy should be stopped immediately
Overdose Effects
Shampoo: Not expected as Ketozole shampoo 2% is intended for external use only. In the event of accidental ingestion, only supportive measures should be carried out. To avoid aspiration, emesis or gastric lavage should not be performed.
Cream: Exaggerated topical application may lead to erythema, oedema and a burning sensation, which will disappear upon discontinuation of the treatment. If accidental ingestion of Ketozole 2% w/w cream occurs, no special measures have to be taken.
Cream: Exaggerated topical application may lead to erythema, oedema and a burning sensation, which will disappear upon discontinuation of the treatment. If accidental ingestion of Ketozole 2% w/w cream occurs, no special measures have to be taken.
Therapeutic Class
Drugs for subcutaneous and mycoses
Storage Conditions
Keep below 25°C temperature, away from light & moisture. Keep out of the reach of children.
Chemical Structure
| Molecular Formula : | C26H28Cl2N4O4 |
| Chemical Structure : | 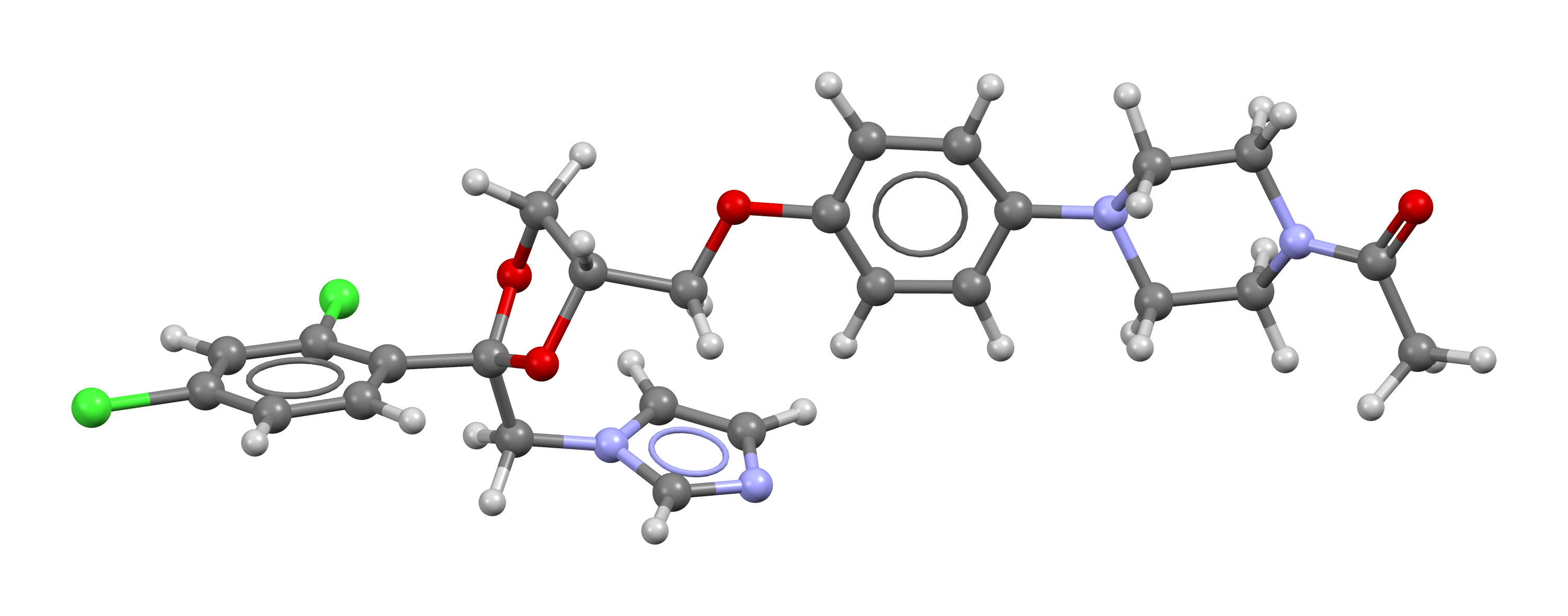 |
Common Questions about Ketozole 2% Medicated Bar
What is Ketozole 2% Medicated Bar?
Ketozole 2% Medicated Bar belongs to the class of drugs called azole antifungals. It works by stopping the growth of the fungus.
What is Ketozole 2% Medicated Bar used for?
Ketozole 2% Medicated Bar is an antiandrogen, antifungal, and antiglucocorticoid medication used to treat a number of fungal infections.


