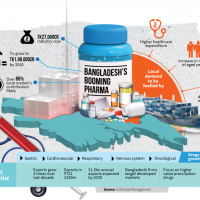
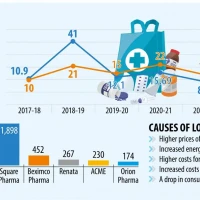
Ten new antibiotics for resistant infections nearing release
05 Nov, 2017

IDWeek Chair Person David Andes said that he did not remember a previous time when so many new drugs were either close to or had received FDA approval
Ten new antibiotics for the treatment of resistant strains of bacterial infections have either received or are nearing approval from the US Food and Drug Administration (FDA). Details on these upcoming drugs were presented at IDWeek 2017 in San Diego, California, in early October. IDWeek Chair Person David Andes said that he did not remember a previous time when so many new drugs were either close to or had received FDA approval, reports Medscape.
The antibiotics were:
Meropenem and Vaborbactum: To be taken as a fixed-dose combination, Meropenem and Vaborbactum were recently approved by FDA for the treatment of complicated urinary tract infections in adults or acute pyelonephritis, an infection of the kidney. The antibiotics were granted FDA approval eight years after beginning laboratory research.
Delafloxacin: Developed to treat bacterial skin and skin structure infections, Delafloxacin was certified by the FDA as a “qualified infectious disease product” in June this year. The antibiotic comes in two different forms, for either intravenous or oral intake.
The drug is currently in a phase 3 trial of community-acquired bacterial pneumonia.
Lefamulin: Another antibiotic for the treatment of community-acquired bacterial pneumonia, Lefamulin yielded strong results during its phase 3 trials. The antibiotic has also shown effectiveness against a number of sexually transmitted infections.
Fosfomycin: Fosfomycin has already been in use in Europe for over 45 years. The FDA has granted the injected antibiotic Fast Track and Qualified Infectious Disease Product status for demonstrating effectiveness against complicated urinary tract infections, hospital-acquired bacterial pneumonia, ventilator-associated bacterial pneumonia and complicated intra-abdominal infections.
A new application for the drug is set to be submitted early next year
Cefiderocol: Cefiderocol is in the late stage of development, and has shown promise in dealing with Gram-negative pathogens, including highly-resistant forms of pseudomonas aeruginosa, acinetobactor baumanii and enterobacteriaceae.
Plazomicin: This antibiotic is undergoing testing in phase 3 for the treatment of two serious bacterial infections caused by multidrug-resistant enterobacteriaceae. The antibiotic could in future be used to treat a variety of bloodstream infections as well as complicated urinary tract infections or acute pyelonephritis.
Omadacycline: Omadacycline is also in late stage development for the treatment of community-acquired bacterial pneumonia, as well as skin and skin structure infections.
Iclaprim: This antibiotic was developed combat trimethoprim-resistant bacteria. Unlike vancomycin, which is a standard antibiotic used to treat Gram-positive infections, there is no evidence of nephrotoxicity with iclaprim.
Relebactum: Currently undergoing phase 3 trials, relebactum can be used to treat Gram-negative bacteria that are resistant to established antibiotic imipenem.
Eravacycline: Eravacycline has also been granted Fast Track and Qualified Infectious Disease Product status for showing promise in dealing with complicated intra-abdominal infections. The antibiotic comes in both injected and orally consumed forms.
Source: dhakatribune.com
Ten new antibiotics for the treatment of resistant strains of bacterial infections have either received or are nearing approval from the US Food and Drug Administration (FDA). Details on these upcoming drugs were presented at IDWeek 2017 in San Diego, California, in early October. IDWeek Chair Person David Andes said that he did not remember a previous time when so many new drugs were either close to or had received FDA approval, reports Medscape.
The antibiotics were:
Meropenem and Vaborbactum: To be taken as a fixed-dose combination, Meropenem and Vaborbactum were recently approved by FDA for the treatment of complicated urinary tract infections in adults or acute pyelonephritis, an infection of the kidney. The antibiotics were granted FDA approval eight years after beginning laboratory research.
Delafloxacin: Developed to treat bacterial skin and skin structure infections, Delafloxacin was certified by the FDA as a “qualified infectious disease product” in June this year. The antibiotic comes in two different forms, for either intravenous or oral intake.
The drug is currently in a phase 3 trial of community-acquired bacterial pneumonia.
Lefamulin: Another antibiotic for the treatment of community-acquired bacterial pneumonia, Lefamulin yielded strong results during its phase 3 trials. The antibiotic has also shown effectiveness against a number of sexually transmitted infections.
Fosfomycin: Fosfomycin has already been in use in Europe for over 45 years. The FDA has granted the injected antibiotic Fast Track and Qualified Infectious Disease Product status for demonstrating effectiveness against complicated urinary tract infections, hospital-acquired bacterial pneumonia, ventilator-associated bacterial pneumonia and complicated intra-abdominal infections.
A new application for the drug is set to be submitted early next year
Cefiderocol: Cefiderocol is in the late stage of development, and has shown promise in dealing with Gram-negative pathogens, including highly-resistant forms of pseudomonas aeruginosa, acinetobactor baumanii and enterobacteriaceae.
Plazomicin: This antibiotic is undergoing testing in phase 3 for the treatment of two serious bacterial infections caused by multidrug-resistant enterobacteriaceae. The antibiotic could in future be used to treat a variety of bloodstream infections as well as complicated urinary tract infections or acute pyelonephritis.
Omadacycline: Omadacycline is also in late stage development for the treatment of community-acquired bacterial pneumonia, as well as skin and skin structure infections.
Iclaprim: This antibiotic was developed combat trimethoprim-resistant bacteria. Unlike vancomycin, which is a standard antibiotic used to treat Gram-positive infections, there is no evidence of nephrotoxicity with iclaprim.
Relebactum: Currently undergoing phase 3 trials, relebactum can be used to treat Gram-negative bacteria that are resistant to established antibiotic imipenem.
Eravacycline: Eravacycline has also been granted Fast Track and Qualified Infectious Disease Product status for showing promise in dealing with complicated intra-abdominal infections. The antibiotic comes in both injected and orally consumed forms.
Source: dhakatribune.com