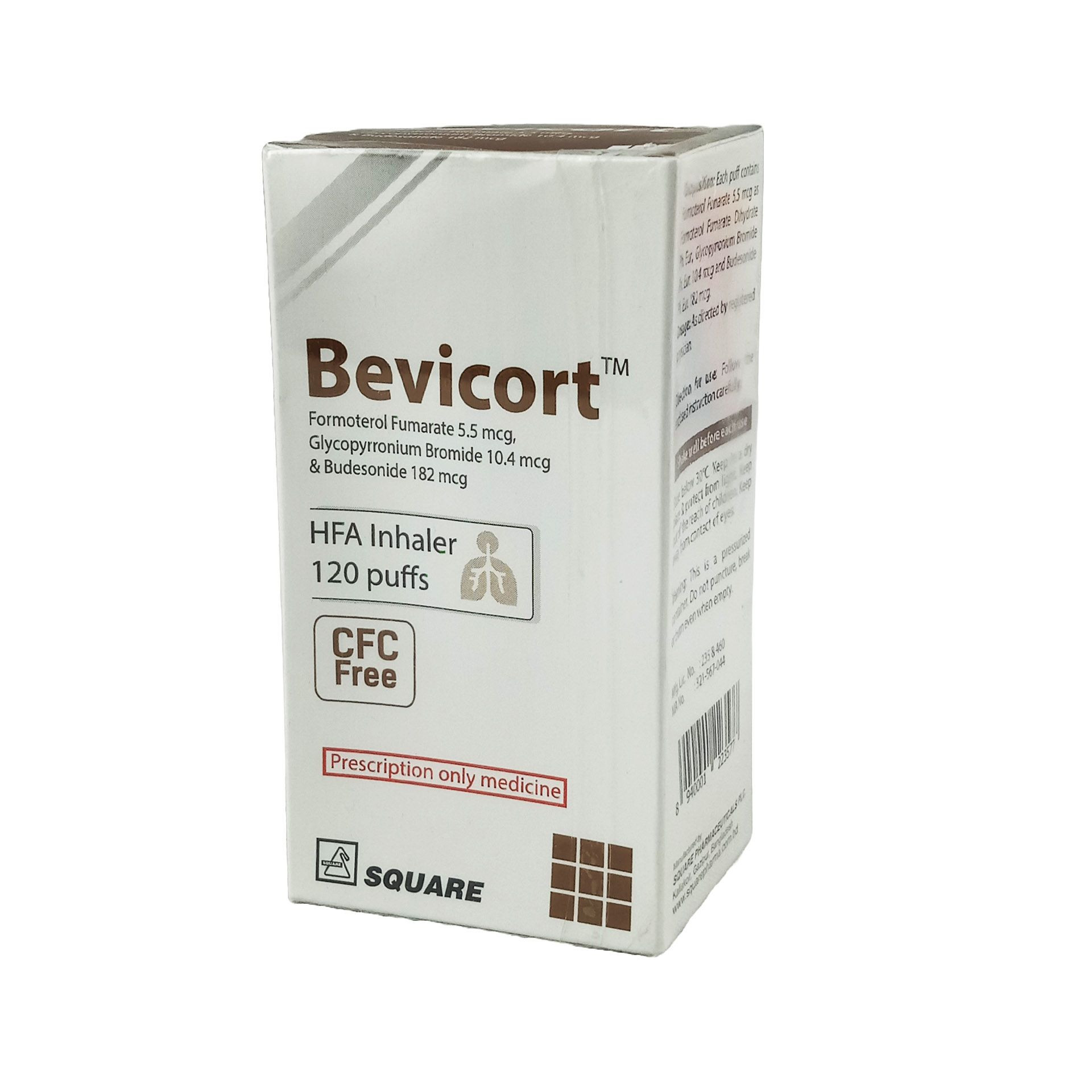
Bevicort Metered-Dose Inhaler (MDI)
Pack Image
(5.5 mcg+10.4 mcg+182 mcg)/actuation
120 metered sprays:
৳ 1,200.00
Indications
Bevicort inhaler is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). This Inhaler is not indicated for the relief of acute bronchospasm or for the treatment of asthma.
Composition
Each puff contains-
- Formoterol Fumarate 5.5 mcg as Formoterol Fumarate Dihydrate Ph. Eur.
- Glycopyrronium Bromide Ph. Eur. 10.4 mcg
- Budesonide Ph. Eur. 182 mcg
Pharmacology
This Inhaler is a pressurized metered-dose inhaler that delivers a combination of micronized Budesonide, an inhaled corticosteroid (ICS), micronized glycopyrrolate (an anticholinergic), and micronized formoterol fumarate, an inhaled long-acting beta2-adrenergic agonist (a LABA), for oral inhalation. ICS medicines such as budesonide help to decrease inflammation in the lungs. Inflammation in the lungs can lead to breathing problems. Anticholinergic medicines, such as glycopyrrolate, and LABA medicines, such as formoterol fumarate help the muscles around the airways in the lungs stay relaxed to prevent symptoms, such as wheezing, cough, chest tightness, and shortness of breath.
Dosage & Administration
The recommended dosage of is two inhalations twice daily, once in the morning and again in the evening, by oral inhalation. Do not take more than two inhalations twice daily. After inhalation, rinse mouth with water without swallowing
Interaction
- Strong cytochrome P450 3A4 inhibitors (e.g. ritonavir): Use with caution. May cause systemic corticosteroid effects.
- Other adrenergic drugs may potentiate effect: Use with caution.
- Diuretics, xanthine derivatives or steroids may potentiate hypokalemia or ECG changes. Use with caution.
- Monoamine oxidase inhibitors and tricyclic antidepressants: Use with extreme caution. May potentiate effect of formoterol fumarate on cardiovascular system.
- Beta-blockers: Use with caution. May block bronchodilatory effects of beta-agonists and produce severe bronchospasm.
- Anticholinergics: May interact additively with concomitantly used anticholinergic medications. Avoid administration of this spray with other anticholinergic-containing drugs.
Contraindications
This is contraindicated in patients who have demonstrated hypersensitivity to budesonide, glycopyrrolate, formoterol, or any of the excipients.
Side Effects
Serious asthma-related events-hospitalizations, intubations, death, Candida albicans infection, increased risk of pneumonia, immunosuppression and risk of infections, hypercorticism and adrenal suppression, paradoxical bronchospasm, hypersensitivity reactions including anaphylaxis, cardiovascular effects, reduction in bone mineral density, worsening of narrow-angle glaucoma and cataracts, worsening of urinary retention
Pregnancy & Lactation
There are no adequate and well-controlled studies with this inhaler or with two of its individual components, Glycopyrrolate or Formoterol Fumarate, in pregnant women to inform a drug-associated risk. There are no available data on the effects of this inhaler on the breastfed child or on milk production. Budesonide, like other ICS, is present in human milk. There are no available data on the presence of Glycopyrrolate or Formoterol Fumarate in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for this inhaler and any potential adverse effects on the breastfed child from this inhaler or from the underlying maternal condition.
Precautions & Warnings
The safety and efficacy of this inhaler in patients with asthma have not been established. this inhaler is not indicated for the treatment of asthma. This inhaler has not been studied in patients with acutely deteriorating COPD. The use of this inhaler in this setting is not appropriate. This inhaler should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. This inhaler has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled short-acting beta2-agonist. When beginning treatment with this inhaler, patients who have been taking inhaled, short-acting beta2-agonists on a regular basis (e.g., four times a day) should be instructed to discontinue the regular use of these drugs and use them only for symptomatic relief of acute respiratory symptoms. When prescribing this inhaler, the healthcare provider should also prescribe an inhaled, short acting beta 2-agonist and instruct the patient on how it should be used. Increasing inhaled beta 2-agonist use is a signal of deteriorating disease for which prompt medical attention is indicated.
The daily dosage of this inhaler should not be increased beyond the recommended dose. As with other inhaled drugs containing beta2-adrenergic agents, this inhaler should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
Patients using this inhaler should not use another medicine containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason.
this inhaler contains budesonide, an ICS. Localized infections of the mouth and pharynx with Candida albicans have occurred in subjects treated with orally inhaled drug products containing budesonide. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with this inhaler continues.
Lower respiratory tract infections, including pneumonia, have been reported following the inhaled administration of corticosteroids. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of pneumonia and exacerbations frequently overlap.
Patients who are using drugs that suppress the immune system are more susceptible to infection than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids.
Particular care is needed for patients who have been transferred from systemically active corticosteroids to ICS because deaths due to adrenal insufficiency have occurred in patients during and after transfer from systemic corticosteroids to less systemically available ICS. Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible.
Effects of budesonide on the HPA axis are not observed with the therapeutic doses of budesonide in this inhaler. However, exceeding the recommended dosage or co-administration with a strong cytochrome P450 3A4 (CYP3A4) inhibitor may result in HPA dysfunction.
It is possible that systemic corticosteroid effects, such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, appropriate therapy should be initiated as needed.
Caution should be exercised when considering the co-administration of this inhaler with long-term ketoconazole, and other known strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) because adverse effects related to increased systemic exposure to budesonide may occur.
As with other inhaled therapies, this inhaler can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with this inhaler, it should be treated immediately with an inhaled, short-acting bronchodilator; this inhaler should be discontinued immediately and alternative therapy should be instituted.
Immediate hypersensitivity reactions have been reported after administration of Budesonide, Glycopyrrolate or Formoterol Fumarate, the components of this inhaler. If signs suggesting allergic reactions occur, in particular, angioedema (including difficulties in breathing or swallowing, swelling of tongue, lips, and face), urticaria, or skin rash, this inhaler should be stopped at once and alternative treatment should be considered.
Formoterol fumarate, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias, such as supraventricular tachycardia and extrasystoles. If such effects occur, this inhaler may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiographic changes, such as flattening of the Twave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown. Therefore, this inhaler should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension
The daily dosage of this inhaler should not be increased beyond the recommended dose. As with other inhaled drugs containing beta2-adrenergic agents, this inhaler should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
Patients using this inhaler should not use another medicine containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason.
this inhaler contains budesonide, an ICS. Localized infections of the mouth and pharynx with Candida albicans have occurred in subjects treated with orally inhaled drug products containing budesonide. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with this inhaler continues.
Lower respiratory tract infections, including pneumonia, have been reported following the inhaled administration of corticosteroids. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of pneumonia and exacerbations frequently overlap.
Patients who are using drugs that suppress the immune system are more susceptible to infection than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids.
Particular care is needed for patients who have been transferred from systemically active corticosteroids to ICS because deaths due to adrenal insufficiency have occurred in patients during and after transfer from systemic corticosteroids to less systemically available ICS. Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible.
Effects of budesonide on the HPA axis are not observed with the therapeutic doses of budesonide in this inhaler. However, exceeding the recommended dosage or co-administration with a strong cytochrome P450 3A4 (CYP3A4) inhibitor may result in HPA dysfunction.
It is possible that systemic corticosteroid effects, such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, appropriate therapy should be initiated as needed.
Caution should be exercised when considering the co-administration of this inhaler with long-term ketoconazole, and other known strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) because adverse effects related to increased systemic exposure to budesonide may occur.
As with other inhaled therapies, this inhaler can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with this inhaler, it should be treated immediately with an inhaled, short-acting bronchodilator; this inhaler should be discontinued immediately and alternative therapy should be instituted.
Immediate hypersensitivity reactions have been reported after administration of Budesonide, Glycopyrrolate or Formoterol Fumarate, the components of this inhaler. If signs suggesting allergic reactions occur, in particular, angioedema (including difficulties in breathing or swallowing, swelling of tongue, lips, and face), urticaria, or skin rash, this inhaler should be stopped at once and alternative treatment should be considered.
Formoterol fumarate, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias, such as supraventricular tachycardia and extrasystoles. If such effects occur, this inhaler may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiographic changes, such as flattening of the Twave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown. Therefore, this inhaler should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension
Use in Special Populations
Hepatic impairment: Budesonide and formoterol fumarate systemic exposure may increase in patients with severe hepatic impairment. Monitor patients for signs of increased drug exposure.
Renal impairment: In patients with severe renal impairment, use should be considered only if the potential benefit of the treatment outweighs the risk.
Pediatric Use: This is not indicated for use in children. The safety and effectiveness of this spray have not been established in pediatric patients.
Geriatric Use: Based on available data, no adjustment of the dosage of This in geriatric patients is necessary, but greater sensitivity in some older individuals cannot be ruled out.
Renal impairment: In patients with severe renal impairment, use should be considered only if the potential benefit of the treatment outweighs the risk.
Pediatric Use: This is not indicated for use in children. The safety and effectiveness of this spray have not been established in pediatric patients.
Geriatric Use: Based on available data, no adjustment of the dosage of This in geriatric patients is necessary, but greater sensitivity in some older individuals cannot be ruled out.
Overdose Effects
No cases of overdose have been reported with this inhaler; therefore, the risks associated with overdosage for the individual components described below apply to this inhaler. Treatment of overdosage consists of discontinuation of this inhaler together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. Cardiac monitoring is recommended in case of overdosage.
Budesonide: If used at excessive doses for prolonged periods, systemic corticosteroid effects, such as hypercorticism may occur.
Glycopyrrolate: High doses of Glycopyrrolate, a component of this inhaler, may lead to anticholinergic signs and symptoms such as nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure (causing pain, vision disturbances or reddening of the eye), obstipation, or difficulties in voiding.
Formoterol Fumarate: An overdose of Formoterol Fumarate would likely lead to an exaggeration of effects that are typical for beta2-agonists: seizures, angina, hypertension, hypotension, tachycardia, atrial and ventricular tachyarrhythmias, nervousness, headache, tremor, palpitations, muscle cramps, nausea, dizziness, sleep disturbances, metabolic acidosis, hyperglycemia, hypokalemia. As with all sympathomimetic medications, cardiac arrest, and even death may be associated with overdosage of Formoterol Fumarate.
Budesonide: If used at excessive doses for prolonged periods, systemic corticosteroid effects, such as hypercorticism may occur.
Glycopyrrolate: High doses of Glycopyrrolate, a component of this inhaler, may lead to anticholinergic signs and symptoms such as nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure (causing pain, vision disturbances or reddening of the eye), obstipation, or difficulties in voiding.
Formoterol Fumarate: An overdose of Formoterol Fumarate would likely lead to an exaggeration of effects that are typical for beta2-agonists: seizures, angina, hypertension, hypotension, tachycardia, atrial and ventricular tachyarrhythmias, nervousness, headache, tremor, palpitations, muscle cramps, nausea, dizziness, sleep disturbances, metabolic acidosis, hyperglycemia, hypokalemia. As with all sympathomimetic medications, cardiac arrest, and even death may be associated with overdosage of Formoterol Fumarate.
Therapeutic Class
Combined bronchodilators
Storage Conditions
Pressurized canister, do not puncture, break or incinerate even when empty as canister may explode. Avoid exposure to direct sunlight or heat. Clean your inhaler regularly as per direction. Do not store above 30° C. Keep in a dry place. Protect from light and keep out of the reach of children. Keep away from eyes. Discard within three months after removing from the foil pouch. For best results, the canister should be at room temperature before use. Shake well before each use.