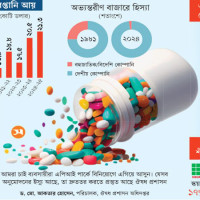
Vonoprazan is now USFDA-approved. (First approved November 1, 2023)
03 Nov, 2023

FDA Approved: Yes (First approved November 1, 2023)
Brand name: Voquezna
Generic name: vonoprazan
Dosage form: Tablets
Company: Phathom Pharmaceuticals, Inc.
Treatment for: Erosive Esophagitis
Voquezna (vonoprazan) is a potassium-competitive acid blocker (P-CAB) for the treatment of erosive esophagitis.
Voquezna is indicated:
Voquezna works by blocking acid secretion in the stomach to help heal all grades of erosive esophagitis and to relieve heartburn.
FDA approval of Voquezna was based on positive results from the randomized, double-blind Phase 3 PHALCON-EE study (NCT04124926) comparing Voquezna to lansoprazole in the healing and maintenance of healing of erosive esophagitis.
Common adverse reactions in patients receiving treatment for healing of erosive esophagitis include gastritis, diarrhea, abdominal distension, abdominal pain, and nausea. Common adverse reactions in patients receiving maintenance treatment for healed erosive esophagitis include gastritis, abdominal pain, dyspepsia, hypertension, and urinary tract infection. Common adverse reactions in patients receiving treatment for H. pylori infection include diarrhea, dysgeusia, vulvovaginal candidiasis, abdominal pain, headache, hypertension, and nasopharyngitis.
In 2022, the FDA approved two combination products containing vonoprazan: Voquezna Triple Pak (vonoprazan, amoxicillin, and clarithromycin) and Voquezna Dual Pak (vonoprazan and amoxicillin) for the treatment of Helicobacter pylori (H. pylori) infection in adults.
Source: drugs.com/history/voquezna.html
Brand name: Voquezna
Generic name: vonoprazan
Dosage form: Tablets
Company: Phathom Pharmaceuticals, Inc.
Treatment for: Erosive Esophagitis
Voquezna (vonoprazan) is a potassium-competitive acid blocker (P-CAB) for the treatment of erosive esophagitis.
Voquezna is indicated:
- for healing of all grades of erosive esophagitis and relief of heartburn associated with erosive esophagitis in adults.
- to maintain healing of all grades of erosive esophagitis and relief of heartburn associated with erosive esophagitis in adults.
- in combination with amoxicillin and clarithromycin for the treatment of Helicobacter pylori (H. pylori) infection in adults.
- in combination with amoxicillin for the treatment of H. pylori infection in adults.
Voquezna works by blocking acid secretion in the stomach to help heal all grades of erosive esophagitis and to relieve heartburn.
FDA approval of Voquezna was based on positive results from the randomized, double-blind Phase 3 PHALCON-EE study (NCT04124926) comparing Voquezna to lansoprazole in the healing and maintenance of healing of erosive esophagitis.
- Voquezna 20 mg (for healing of erosive esophagitis) met the primary endpoint of non-inferiority for complete healing by Week 8 with a healing rate of 93% compared to 85% for lansoprazole 30 mg, with superior rates of healing demonstrated in a secondary endpoint in patients at Week 2 compared to lansoprazole (70% for Voquezna 20 mg and 53% for lansoprazole 30 mg).
- Voquezna 10 mg (for maintenance of healed erosive esophagitis) was superior to lansoprazole 15 mg in maintaining healing at six months in all randomized patients (79% for Voquezna 10 mg, compared to 72% for lansoprazole 15 mg). Voquezna 10 mg also demonstrated non-inferiority to lansoprazole 15 mg for the relief of heartburn in erosive esophagitis patients over a six month period.
- for the healing of erosive esophagitis the dosage is 20 mg once daily for 8 weeks.
- for the maintenance of healed erosive esophagitis the dosage is 10 mg once daily for up to 6 months.
Common adverse reactions in patients receiving treatment for healing of erosive esophagitis include gastritis, diarrhea, abdominal distension, abdominal pain, and nausea. Common adverse reactions in patients receiving maintenance treatment for healed erosive esophagitis include gastritis, abdominal pain, dyspepsia, hypertension, and urinary tract infection. Common adverse reactions in patients receiving treatment for H. pylori infection include diarrhea, dysgeusia, vulvovaginal candidiasis, abdominal pain, headache, hypertension, and nasopharyngitis.
In 2022, the FDA approved two combination products containing vonoprazan: Voquezna Triple Pak (vonoprazan, amoxicillin, and clarithromycin) and Voquezna Dual Pak (vonoprazan and amoxicillin) for the treatment of Helicobacter pylori (H. pylori) infection in adults.
Source: drugs.com/history/voquezna.html