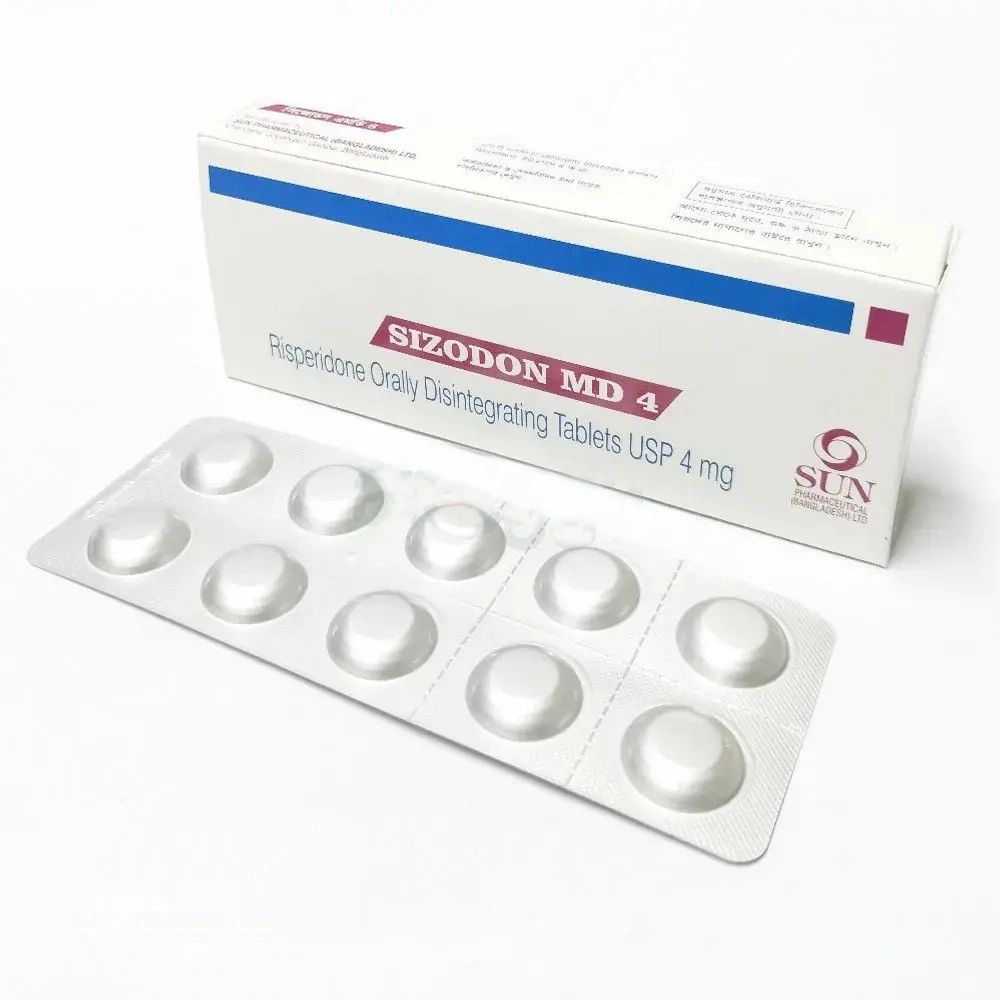
Unit Price:
৳ 9.50
(5 x 10: ৳ 475.00)
Strip Price:
৳ 95.00
Also available as:
Indications
Sizodon MD tablet is indicated for the treatment of-
- Acute and chronic psychoses
- Mania
- Schizophrenia
Pharmacology
Risperidone is a selective monoaminergic antagonist having a high affinity for serotoninergic 5-HT2 and dopaminergic D2 receptors. Risperidone binds also to alpha1 adrenergic receptors and with lower affinity, to H1 histamine and alpha2 adrenergic receptors. Risperidone has no affinity for 8 cholinergic receptors. Risperidone, as a potent D2 antagonist, improves the positive symptoms of schizophrenia but causes less depression of motor activity. Balanced central serotonin and dopamine antagonism may reduce extrapyramidal side effect liability and extend the therapeutic activity to the negative and affective symptoms of schizophrenia.
Dosage & Administration
Psychoses:
- 2 mg in 1-2 divided doses on 1st day then 4 mg in 1-2 divided doses on second day (Slower titration appropriate in some patients);
- Usual dose range 4-6 mg daily; doses above 10 mg daily only if benefit considered to outweigh risk (max. 16 mg daily).
- Elderly (or in hepatic or renal impairment) initially 1 mg daily in two divided doses increased in steps of 1-2 mg twice daily.
- Child under 15 years not recommended.
- Initially, 2 mg once daily increased if necessary in step of 1 mg daily; usual dose range 1-6 mg daily;
- Elderly (or in hepatic or renal impairment) initially 1 mg daily in two divided doses increased in steps of 1-2 mg twice daily.
- Risperidone should be generally administered at 1 mg BID initially, with increases in increments of 1 mg BID on the second and third day, as tolerated, to a target dose of 3 mg BID by the third day.
- Further dosage adjustments, if indicated, should generally occur at intervals of not less than 1 week. When dosage adjustments are necessary, small dose increments/decrements of 1-2 mg are recommended.
Interaction
Sizodon MD May antagonize the effects of levodopa and dopamine agonists. Chronic administration with Carbamazepine reduces plasma clearance of Sizodon MD. Chronic administration with Clozapine may decrease the clearance of Sizodon MD. Sizodon MD may enhance the effects of certain antihypertensives.
Contraindications
Risperidone is contraindicated in patients with a known hypersensitivity to the product.
Side Effects
Insomnia, agitation, anxiety, headache, less commonly drowsiness, impaired concentration, fatigue, blurred vision, constipation, nausea and vomiting, dyspepsia, abdominal pain, hyperprolactinaemia, urine incontinence, tachycardia, hypertension, edema, rash, rhinitis, cerebrovascular accident, neurtropenia and thrombocytopenia have been reported.
Pregnancy & Lactation
Although, in experimental animals, Risperidone did not show direct reproductive toxicity, some indirect, prolactin- and CNS-mediated effects were observed, No teratogenicity effect of Risperidone was noted in any study. The safety of Risperidone for use during human pregnancy has not been established. In animal studies, Risperidone and 9-hydroxyrisperidone are excreted in the milk. It has been demonstrated that Risperidone and 9-hydroxyrisperidone are also excreted in human breast milk. Therefore, women receiving Risperidone should not breastfeed.
Precautions & Warnings
Special precaution should be taken in case of preexisting cardiovascular diseases, discontinue use if signs and symptoms of tardive dyskinesia occur, renal and hepatic impairment, elderly epilepsy, Parkinson's disease and in pregnancy.
Therapeutic Class
Atypical neuroleptic drugs
Storage Conditions
Do not store above 30°C. Keep away from light and out of the reach of children.